 |
Munich, Germany (SPX) Jan 11, 2011 The mechanism for binding oxygen to metalloporphyrins is a vital process for oxygen-breathing organisms. Understanding how small gas molecules are chemically bound to the metal complex is also important in catalysis or the implementation of chemical sensors. When investigating these binding mechanisms, scientists use porphyrin rings with a central cobalt or iron atom. They coat a copper or silver support surface with these substances. An important characteristic of porphyrins is their conformational flexibility. Recent research has shown that each specific geometric configuration of the metalloporphyrins has a distinct influence on their functionality. In line with the current state of research, the scientists expected only a single CO molecule to bind axially to the central metallic atom. However, detailed scanning tunnel microscopy experiments by Knud Seifert revealed that, in fact, two gas molecules dock between the central metallic atom and the two opposite nitrogen atoms. Decisive is the saddle shape of the porphyrin molecules, in which the gas molecules assume the position of the rider. The significance of the saddle geometry became apparent in model calculations done by Marie-Laure Bocquet from the University of Lyon. Her analysis helped the researchers understand the novel binding mode in detail. She also showed that the shape of the molecular saddle remains practically unchanged, even after the two gas molecules bind to the porphyrin. The porphyrins reacted very differently when the researchers replaced the carbon monoxide with stronger-binding nitrogen monoxide. As expected, this binds directly to the central atom, though only a single molecule fits in each porphyrin ring. This has a significant effect on the electronic structure of the carrier molecule, and the characteristic saddle becomes flattened. Thus, the porphyrin reacts very differently to different kinds of gas - a result that is relevant for potential applications, such as sensors. Dr. Willi Auwaerter, one of the authors, is thrilled: "What's new is that we actually saw, for the first time, the mechanism on a molecular level. We even can selectively move individual gas molecules from one porphyrin to another." The team aims to explain the physical and chemical processes on surfaces and in nanostructures. Once these fundamental questions are answered, they will take on new challenges: How big is the influence of the central atom? How does the binding change in planar conformations? How can such systems be utilized to implement catalyzers and sensors through controlled charge transfers? Cis-dicarbonyl binding at cobalt and iron porphyrins with saddle-shape conformation, Knud Seufert, Marie-Laure Bocquet, Willi Auwarter, Alexander Weber-Bargioni, Joachim Reichert, Nicolas Lorente und Johannes V. Barth, Nature Chemistry, Online 9. January 2011 - DOI: 10.1038/NCHEM.956. Discriminative Response of Surface-Confined Metalloporphyrin Molecules to Carbon and Nitrogen Monoxide, Knud Seufert, Willi Auwaerter und Johannes V. Barth, Journal of the American Chemical Society, 2010, 132, 18141? - DOI: 10.1021/ja1054884
Share This Article With Planet Earth
Related Links Technische Universitaet Muenchen Space Technology News - Applications and Research
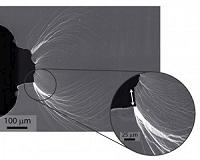 New Glass Tops Steel in Strength and Toughness
New Glass Tops Steel in Strength and ToughnessBerkeley, Germany (SPX) Jan 11, 2011 Glass stronger and tougher than steel? A new type of damage-tolerant metallic glass, demonstrating a strength and toughness beyond that of any known material, has been developed and tested by a collaboration of researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab)and the California Institute of Technology. What's more, even better versions of ... read more |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2010 - SpaceDaily. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |