 |
Washington DC (SPX) Jan 11, 2011 Sophisticated tools allow scientists to subject the basic elements of matter to conditions drastic enough to modify their behavior. By doing this, they can expand our understanding of matter. A research team including three Carnegie scientists was able to demonstrate surprising properties of the element lithium under intense pressure and low temperatures. Their results were published on the Nature Physics website. Lithium is the first metal in the periodic table and is the least dense solid element at room temperature. It is most commonly known for its use in batteries for consumer electronics, such as cell phones and laptop computers. And, with only three electrons per atom, lithium should behave like a model, simple metal. However, this research has shown that under pressure ranging between about 395,000 atmospheres (40 GPa) and about 592,000 atmospheres (60 GPa), lithium behaves in a manner that's anything but simple. Not only does it become a liquid at room temperature, but it then refuses to freeze until the temperature reaches a chilly -115o F. At pressures above about 592,000 atmospheres (60 GPa), when lithium does eventually solidify, it is into a range of highly complex, crystalline states. The highest pressure reached in the study was about 1.3 million atmospheres (130 GPa). The research team, including Malcolm Guthrie, Stanislav Sinogeikin and Ho-kwang (Dave) Mao, of Carnegie's Geophysical Laboratory, believe that this exotic behavior is directly due to the exceptionally low mass of the lithium atom. An elementary result of quantum physics is that atoms continue to move, even when cooled to the lowest possible temperature. As the mass of an atom decreases, the importance of this residual, so called 'zero-point,' energy increases. The researchers speculate that, in the case of lithium, the zero-point energy increases with pressure to the point that melting occurs. This work raises the possibility of uncovering a material which never freezes. The prospect of a metallic liquid at even the lowest temperatures raises the intriguing possibility of an entirely novel material, a superconducting liquid, as proposed previously by theorists for hydrogen at very high pressure.
Share This Article With Planet Earth
Related Links Carnegie Institution Space Technology News - Applications and Research
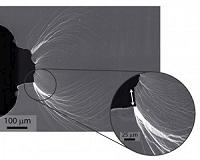 New Glass Tops Steel in Strength and Toughness
New Glass Tops Steel in Strength and ToughnessBerkeley, Germany (SPX) Jan 11, 2011 Glass stronger and tougher than steel? A new type of damage-tolerant metallic glass, demonstrating a strength and toughness beyond that of any known material, has been developed and tested by a collaboration of researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab)and the California Institute of Technology. What's more, even better versions of ... read more |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2010 - SpaceDaily. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |